Difference between revisions of "Microscopy: Fluorescent Resonance Energy Transfer (FRET) Bleed Through and Efficiency"
m |
m |
||
| Line 43: | Line 43: | ||
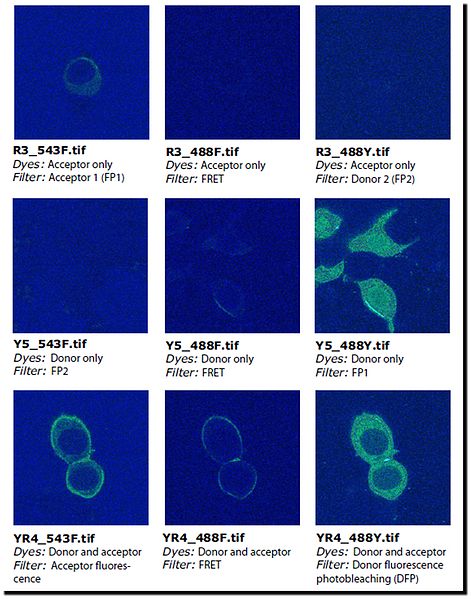
To obtain FRET efficiency, you need, therefore, a set of nine images. Table 2 and Figure 77 list and show the images that are used as examples in this discussion. | To obtain FRET efficiency, you need, therefore, a set of nine images. Table 2 and Figure 77 list and show the images that are used as examples in this discussion. | ||
| − | [[File:FretOptions.jpg|761px|thumb| | + | [[File:FretOptions.jpg|761px|thumb|center|To obtain the FRET efficiency, you need to have a complete set of nine images modeled after the images listed here. The naming convention for these 9 images is as follows: R = Red (acceptor dye), Y = Yellow (donor dye), F = Filter for detecting acceptor signal, Y after number = Filter for detecting donor signal, 488 = Excitation wavelength (nm) for donor dye, 543 = Excitation wavelength (nm) for acceptor dye]] |
<br /> | <br /> | ||
| Line 49: | Line 49: | ||
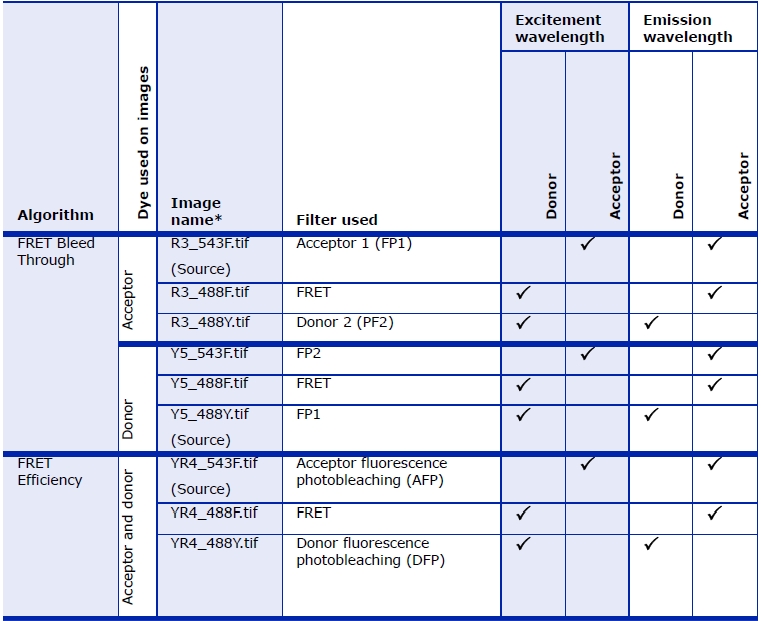
FP stands for "fluorescence protein". The term "FP1 filter" refers to the pair of filters normally used to acquire an image of fluorescence protein 1 (FP1). The pair of filters consists of an excitation filter and an emission filter. In order to measure bleed through, it is necessary to image each of the proteins (1 and 2) with the correct filters (1 and 2), wrong filters (i.e., 2 and 1), as well as image each of the proteins with the FRET filter. The FRET filter uses the excitation filter for the donor protein and the emission filter for the acceptor protein. For the bleed-through calculations I avoided using the terms "donor" and "acceptor" and instead use "1" and "2" since the donor and acceptor are used equivalently for the bleed through calculations. In the second part of the calculation to calculate the FRET efficiency, it does make a difference which is the donor and which is the acceptor, therefore I use the terms DFP and AFP. | FP stands for "fluorescence protein". The term "FP1 filter" refers to the pair of filters normally used to acquire an image of fluorescence protein 1 (FP1). The pair of filters consists of an excitation filter and an emission filter. In order to measure bleed through, it is necessary to image each of the proteins (1 and 2) with the correct filters (1 and 2), wrong filters (i.e., 2 and 1), as well as image each of the proteins with the FRET filter. The FRET filter uses the excitation filter for the donor protein and the emission filter for the acceptor protein. For the bleed-through calculations I avoided using the terms "donor" and "acceptor" and instead use "1" and "2" since the donor and acceptor are used equivalently for the bleed through calculations. In the second part of the calculation to calculate the FRET efficiency, it does make a difference which is the donor and which is the acceptor, therefore I use the terms DFP and AFP. | ||
| − | [[File:FretNineImages.jpg|471px|thumb| | + | [[File:FretNineImages.jpg|471px|thumb|center| Nine example images used to calculate FRET efficiency]] |
<br /> | <br /> | ||
Revision as of 15:24, 31 July 2012
This page is a stub.
Refer to thh HTML version of this MIPAV algorithm http://mipav.cit.nih.gov/documentation/HTML%20Algorithms/MicroscopyFRETBleedThroughEfficiency.html
Summary
This section provides information on and discusses how to use the following two FRET algorithms:
- FRET Bleed Through algorithm
- FRET Efficiency algorithm
Used consecutively, the FRET Bleed Through algorithm uses two sets of three 2D images and the FRET Efficiency algorithm uses one set of three 2D images to measure effects dependent on the proximity of fluorescent-labeled molecules.
You must first run the FRET Bleed Through algorithm twice: once on acceptor-dyed images and once on donor-dyed images. Using the results achieved from running this algorithm, you then use the FRET Efficiency algorithm to process images that were dyed with both the donor and acceptor dyes to obtain the FRET efficiency.
Background
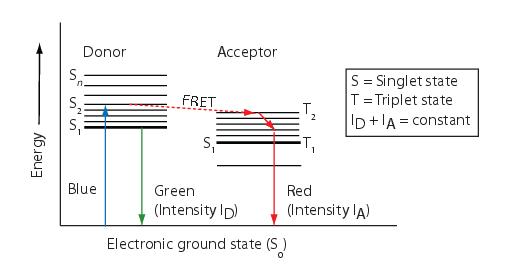
Fluorescent resonance energy transfer (FRET) refers to the non-radiative transfer of energy from an excited fluorochrome, called a donor, to a nearby fluorescent molecule, called an acceptor. The FRET technique measures the fluorescence signals of the donor, the acceptor, and the FRET signal. If FRET occurs, the donor channel signal is quenched and the acceptor channel signal is sensitized or increased.
The energy transfer efficiency E is conventionally defined as the number of energy transfer events divided by the number of photons absorbed by the donor, is related to the distance R between the acceptor and donor by:
<math>
E= \frac{1}{\left [ 1 + \frac{r}{R_0}^6 \right ]}
</math>
where R_0, the Forster critical distance, is the distance at which E = 0.5.
Because FRET falls off as the sixth power of the distance between the donor and the acceptor, virtually no FRET occurs for distances greater than . Since is on the order of 10 to 70 Angstroms, by performing FRET measurements it is possible to distinguish proteins that are merely nearby in the same compartment from those proteins that are interacting with each other. Virtually no FRET occurs for distances that are greater than .
Figure below schematically illustrates the process of fluorescence and FRET. When a fluorescent molecule (donor) is excited (using a blue photon in this example), the molecule goes into an excited state (S1) for a few nanoseconds. During this time the molecule loses some of its energy after which it returns to the ground state (S0) by emission of a less energetic photon (green photon in this example). If another fluorescence molecule (acceptor), which is excited by green photons and emits red photons, is very close by, then it is likely that the donor non-radiatively transfers its excitation energy to the acceptor and returns to the ground state without emitting a photon. As a consequence, the acceptor becomes excited and later returns to the ground state by giving off a photon of lower energy (red photon in this example).
Measuring FRET using the sensitized emission method requires three sets of 2D images. You must first run the FRET Bleed Through algorithm once on three donor dye only images and once on three acceptor dye only images. You then run the FRET Efficiency algorithm on three images with both donor and acceptor dyes.
These images are:
- Image donor and/or acceptor dyes taken with a donor filter
- Image with donor and/or acceptor dyes taken with a FRET filter
- Image with donor and/or acceptor dyes taken with an acceptor filter
To obtain FRET efficiency, you need, therefore, a set of nine images. Table 2 and Figure 77 list and show the images that are used as examples in this discussion.
FP stands for "fluorescence protein". The term "FP1 filter" refers to the pair of filters normally used to acquire an image of fluorescence protein 1 (FP1). The pair of filters consists of an excitation filter and an emission filter. In order to measure bleed through, it is necessary to image each of the proteins (1 and 2) with the correct filters (1 and 2), wrong filters (i.e., 2 and 1), as well as image each of the proteins with the FRET filter. The FRET filter uses the excitation filter for the donor protein and the emission filter for the acceptor protein. For the bleed-through calculations I avoided using the terms "donor" and "acceptor" and instead use "1" and "2" since the donor and acceptor are used equivalently for the bleed through calculations. In the second part of the calculation to calculate the FRET efficiency, it does make a difference which is the donor and which is the acceptor, therefore I use the terms DFP and AFP.
FRET Bleed Through Algorithm
The FRET Bleed Through algorithm requires the six types of images listed in the Table below. You need to run the algorithm twice: Once on the set of three images with acceptor dye only (the first three images listed in the table); and once on the set of three images with donor dye only (the last three images listed in the table).
Sets of images<a name="wp1029914"> </a> |
Example image*<a name="wp1029916"> </a> |
Type of image<a name="wp1029918"> </a> |
|---|---|---|
Acceptor only dyed images<a name="wp1029920"> </a> |
R3_543F.tif<a name="wp1029922"> </a> |
An acceptor only dyed image taken with an acceptor filter set<a name="wp1029924"> </a> |
R3_488F.tif<a name="wp1029928"> </a> |
An acceptor only dyed image taken with a FRET filter set<a name="wp1029930"> </a> |
|
R3_488Y.tif<a name="wp1029934"> </a> |
An acceptor only dyed image taken with a donor filter set<a name="wp1029936"> </a> |
|
Donor only dyed images<a name="wp1029938"> </a> |
Y5_543F.tif<a name="wp1029940"> </a> |
An donor only dyed image taken with an acceptor filter set<a name="wp1029942"> </a> |
Y5_488F.tif<a name="wp1029946"> </a> |
An donor only dyed image taken with a FRET filter set<a name="wp1029948"> </a> |
|
Y5_488Y.tif<a name="wp1029952"> </a> |
A donor only dyed image taken with a donor filter set<a name="wp1029954"> </a> |
|
*Refer to <a href="MicroscopyFRETBleedThroughEfficiency.html#wp1038603">Figure 77</a> to view these example images. <a name="wp1029963"> </a>
| ||